Connect With a Global Community of Cell Therapy Experts
Unlike any other forum, the 9th CAR TCR Summit Europe unites developers from across Europe and Asia, laser focused on advancing CAR and TCR based cell therapies.
With an end-to-end coverage of topics from science to strategy expect to meet C-level executives from biotechs and decision makers across business development, regulatory, preclinical, translation, clinical, analytical, CMC, manufacturing, and market access functions.
This forum gives you the unique opportunity to tailor your own spotlight whether it’s a presentation, panel discussion participation, booth presence, private lunch and more in front of the top names in CAR and TCR.
![CB-CDx-0330-min-scaled11[1] CB-CDx-0330-min-scaled11[1]](https://cartcr-europe.com/wp-content/uploads/sites/229/2024/06/CB-CDx-0330-min-scaled111.jpg)
What to Expect?
Expand Your Reach
Access an audience spanning biotech, academia, and pharma, all focused on accelerating CAR/TCR development and commercialisation
Demonstrate Your Expertise
Position your technologies and services directly to the companies actively seeking support in CAR and TCR R&D, manufacturing, scale-up and more
Nurture Business Relations
Build meaningful partnerships through roundtables, private lunches, and structured networking with C-suite, directors, and technical leads
Key Services & Solutions
- GMP Manufacturing & Tech Transfer: Support with scalable processes, point-of-care manufacturing, and smooth tech transfer to maintain product quality
- Regulatory Strategy & Compliance: Guiding navigation of MHRA, EMA, and FDA expectations
- Clinical Trial Enablement: Assisting with patient enrolment, site logistics, and trial execution for complex CAR/TCR protocols
- Equipment & Platforms to Scale Cell Production: Providing bioreactors, automation systems, and technologies to enable efficient, high-quality expansion of CAR and TCR cell therapies
- Data Management & AI-Driven Insights: Supporting the collection, integration, and analysis of preclinical and clinical data to optimise CAR and TCR design, manufacturing, and patient outcomes
![CB-CDx-0690-min-scaled11[1] CB-CDx-0690-min-scaled11[1]](https://cartcr-europe.com/wp-content/uploads/sites/229/2024/06/CB-CDx-0690-min-scaled111.jpg)
The Top Trailblazers in CAR-TCR Need Your Help with:
GMP Manufacturing & Tech Transfer: Support with scalable processes, point-of-care manufacturing, and smooth tech transfer to maintain product quality
Regulatory Strategy & Compliance: Guiding navigation of MHRA, EMA, and FDA expectations
Clinical Trial Enablement: Assisting with patient enrolment, site logistics, and trial execution for complex CAR/TCR protocols.
Equipment & Platforms to Scale Cell Production: Providing bioreactors, automation systems, and technologies to enable efficient, high-quality expansion of CAR and TCR cell therapies
Data Management & AI-Driven Insights: Supporting the collection, integration, and analysis of preclinical and clinical data to optimize CAR and TCR design, manufacturing, and patient outcomes.
Audience Composition
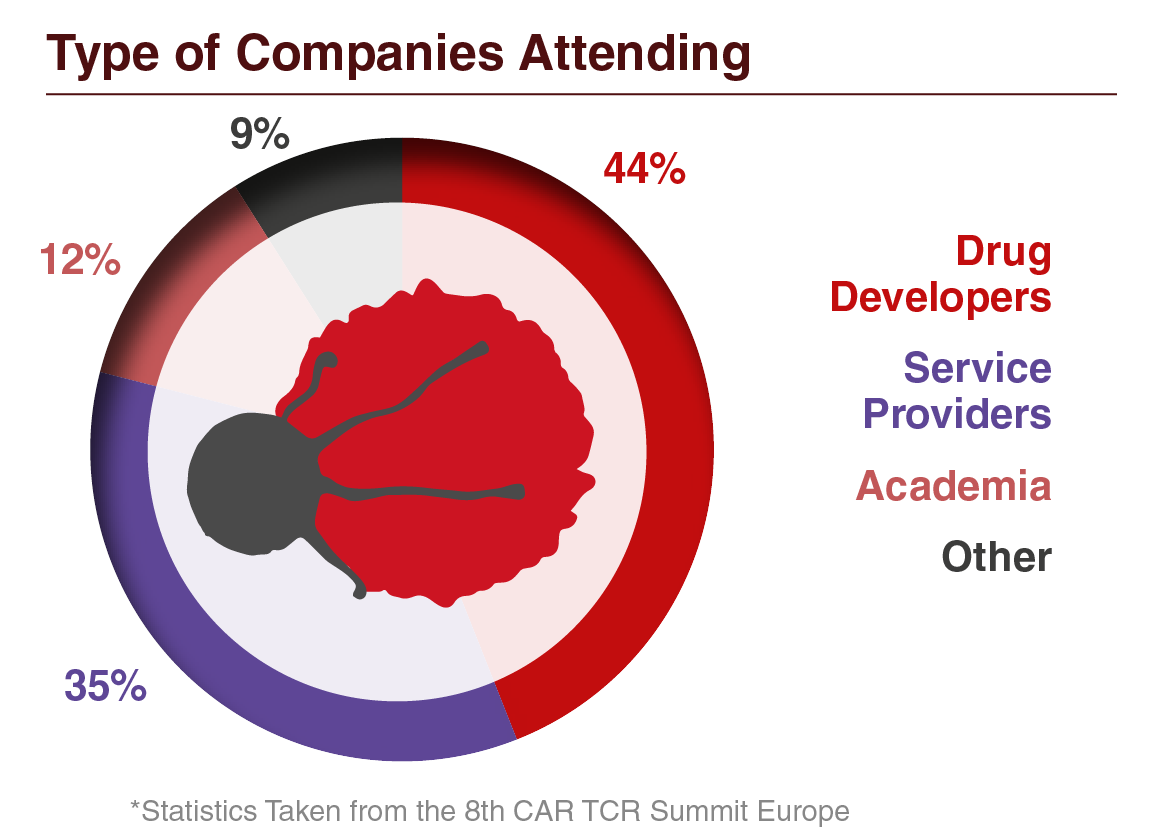
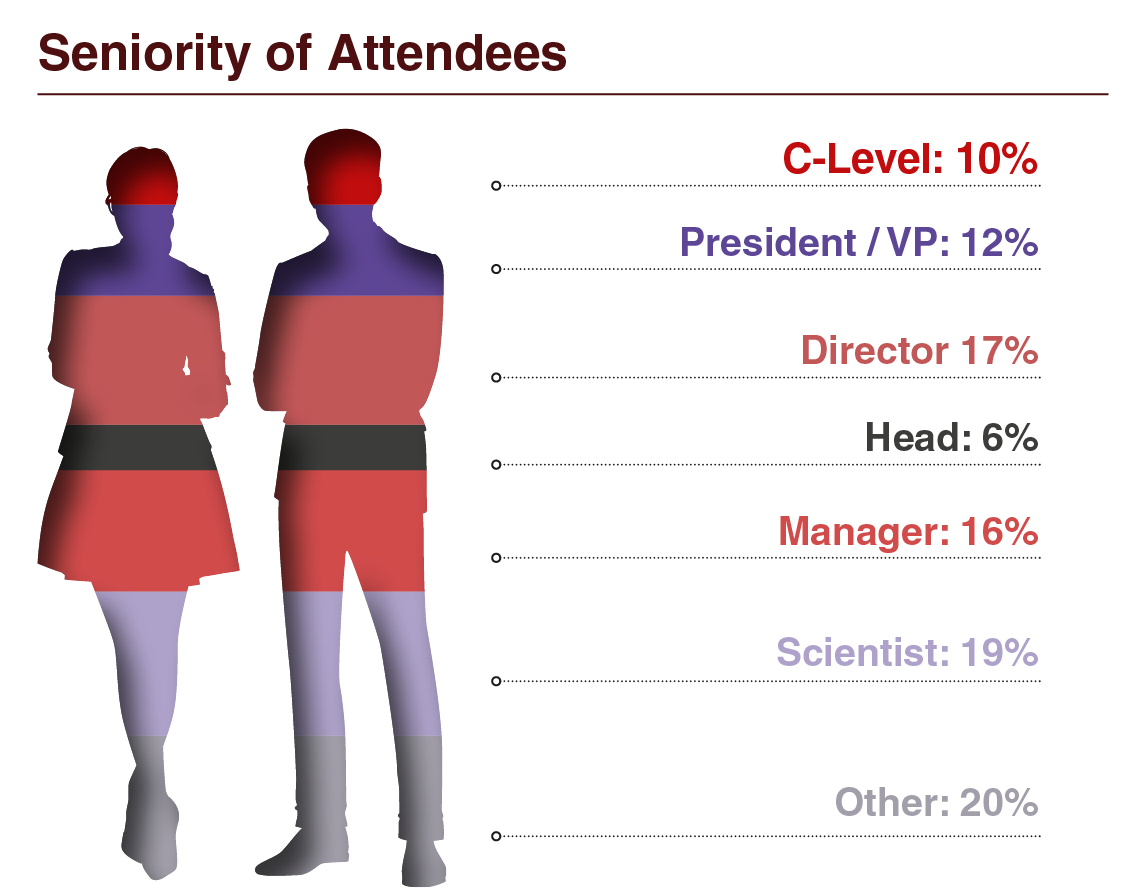
Attending Companies Include

Get in Touch
Take advantage of our bespoke sponsorship opportunities to achieve your commercial goals. Email us if you would like to get involved and discuss a bespoke package suited to your needs.

Kieran Crewe
Senior Business Development Manager

Charlotte Hodgson
Senior Business Development Manager
![CB-CDx-0330-min-scaled11[1] CB-CDx-0330-min-scaled11[1]](https://cartcr-europe.com/wp-content/uploads/sites/229/2024/06/CB-CDx-0330-min-scaled111.jpg)
Maintain Your Competitive Edge
Position yourself alongside other leading solution providers to ensure your brand is at the heart of biopharma discussions.
![CB-CDx-0690-min-scaled11[1] CB-CDx-0690-min-scaled11[1]](https://cartcr-europe.com/wp-content/uploads/sites/229/2024/06/CB-CDx-0690-min-scaled111.jpg)
Network With Decision-Makers
Speak with our audience of senior scientific experts during dedicated meeting breaks and structured networking opportunities.
![CB-CDx-0107-min-scaled11[1] CB-CDx-0107-min-scaled11[1]](https://cartcr-europe.com/wp-content/uploads/sites/229/2025/10/CB-CDx-0107-min-scaled111.jpg)
Showcase Your Capabilities
Explore our bespoke sponsorship options including branding, presenting, and exhibiting to build a package tailored to you and your company's goals.
